 |
PlantRegMap/PlantTFDB v5.0
Plant Transcription
Factor Database
|
| Home TFext BLAST Prediction Download Help About Links PlantRegMap |
Transcription Factor Information
| Basic Information? help Back to Top | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TF ID | Prupe.1G122800.3.p | ||||||||
| Organism | |||||||||
| Taxonomic ID | |||||||||
| Taxonomic Lineage |
cellular organisms; Eukaryota; Viridiplantae; Streptophyta; Streptophytina; Embryophyta; Tracheophyta; Euphyllophyta; Spermatophyta; Magnoliophyta; Mesangiospermae; eudicotyledons; Gunneridae; Pentapetalae; rosids; fabids; Rosales; Rosaceae; Maloideae; Amygdaleae; Prunus
|
||||||||
| Family | CAMTA | ||||||||
| Protein Properties | Length: 873aa MW: 98149 Da PI: 6.9774 | ||||||||
| Description | CAMTA family protein | ||||||||
| Gene Model |
|
||||||||
| Signature Domain? help Back to Top | |||||||
|---|---|---|---|---|---|---|---|
| No. | Domain | Score | E-value | Start | End | HMM Start | HMM End |
| 1 | CG-1 | 135.3 | 2.1e-42 | 15 | 100 | 33 | 118 |
CG-1 33 rpksgsliLynrkkvryfrkDGyswkkkkdgktvrEdhekLKvggvevlycyYahseenptfqrrcywlLeeelekivlvhylevk 118
+ + g+++L++rk++r+frkDG++wkkkkdgktv+E+he+LKvg+ e +++yYah+e+ ptf rrcywlL+++le+ivlvhy+e++
Prupe.1G122800.3.p 15 TMQGGTIVLFDRKMLRNFRKDGHNWKKKKDGKTVKEAHEHLKVGNEERIHVYYAHGEDSPTFVRRCYWLLDKSLEHIVLVHYRETQ 100
5689*******************************************************************************986 PP
| |||||||
| Protein Features ? help Back to Top | ||||||
|---|---|---|---|---|---|---|
| Database | Entry ID | E-value | Start | End | InterPro ID | Description |
| SMART | SM01076 | 7.4E-46 | 1 | 100 | IPR005559 | CG-1 DNA-binding domain |
| PROSITE profile | PS51437 | 60.918 | 1 | 105 | IPR005559 | CG-1 DNA-binding domain |
| Pfam | PF03859 | 9.8E-36 | 17 | 98 | IPR005559 | CG-1 DNA-binding domain |
| SuperFamily | SSF81296 | 1.26E-13 | 324 | 411 | IPR014756 | Immunoglobulin E-set |
| CDD | cd00204 | 5.23E-14 | 484 | 618 | No hit | No description |
| Gene3D | G3DSA:1.25.40.20 | 7.7E-17 | 508 | 621 | IPR020683 | Ankyrin repeat-containing domain |
| Pfam | PF12796 | 5.0E-7 | 509 | 588 | IPR020683 | Ankyrin repeat-containing domain |
| SuperFamily | SSF48403 | 4.51E-17 | 521 | 621 | IPR020683 | Ankyrin repeat-containing domain |
| PROSITE profile | PS50297 | 15.777 | 526 | 630 | IPR020683 | Ankyrin repeat-containing domain |
| PROSITE profile | PS50088 | 11.621 | 559 | 591 | IPR002110 | Ankyrin repeat |
| SMART | SM00248 | 9.2E-6 | 559 | 588 | IPR002110 | Ankyrin repeat |
| SMART | SM00248 | 1500 | 598 | 628 | IPR002110 | Ankyrin repeat |
| PROSITE profile | PS50096 | 7.73 | 707 | 733 | IPR000048 | IQ motif, EF-hand binding site |
| SMART | SM00015 | 26 | 722 | 744 | IPR000048 | IQ motif, EF-hand binding site |
| PROSITE profile | PS50096 | 7.053 | 723 | 752 | IPR000048 | IQ motif, EF-hand binding site |
| SMART | SM00015 | 0.022 | 745 | 767 | IPR000048 | IQ motif, EF-hand binding site |
| PROSITE profile | PS50096 | 10.164 | 746 | 770 | IPR000048 | IQ motif, EF-hand binding site |
| Pfam | PF00612 | 7.7E-4 | 747 | 767 | IPR000048 | IQ motif, EF-hand binding site |
| SMART | SM00015 | 46 | 825 | 847 | IPR000048 | IQ motif, EF-hand binding site |
| PROSITE profile | PS50096 | 7.199 | 826 | 855 | IPR000048 | IQ motif, EF-hand binding site |
| Gene Ontology ? help Back to Top | ||||||
|---|---|---|---|---|---|---|
| GO Term | GO Category | GO Description | ||||
| GO:0005634 | Cellular Component | nucleus | ||||
| GO:0003677 | Molecular Function | DNA binding | ||||
| GO:0005515 | Molecular Function | protein binding | ||||
| Sequence ? help Back to Top |
|---|
| Protein Sequence Length: 873 aa Download sequence Send to blast |
MEKQLGGSEI HGFHTMQGGT IVLFDRKMLR NFRKDGHNWK KKKDGKTVKE AHEHLKVGNE 60 ERIHVYYAHG EDSPTFVRRC YWLLDKSLEH IVLVHYRETQ ELQGSPVTPV NSNNSSSVSD 120 PSAPWLLSEE LDSGANTTFC AGENELSEPG DGLTVKNHEK RLHDINTLEW EELLITNDSK 180 GDIVSCYDQQ NQVVGNGFIS GGASVISAEM SAFGNLTNPT LRSDDVQFNL PDSPYVPTVE 240 YDVNSNVQIR DSIAKTTCDS LDVLVNDGLH SQDSFGRWIN QVMADPPGSV EDPALESSSI 300 AAQNSFASPS ADHLQSSIPH QIFNITDLSP AWAFSNEKTK ILITGFFHQE YLHLAKSDLL 360 CICGDVCLRA EIVQAGVYRC FVPPHLPRVV NLFMSIDGHK PISLVLNFEY RAPVLSDPII 420 SSEENNWEEF QAQMRLAYLL FSSSKSLNIV SNKVSLNALK EAKKFSHRTS HISNSWACLM 480 KAVEDKKSPL PLAKDGLFEL ILKNRLKDWL LEKVVDSSTT KEYDAYGQGV IHLCAILEYT 540 WAVRLFSWSG LSLDFRDRRG WTALHWAAYC GREKMVAVLL SAGAKPNLVT DPSSENPGGY 600 TAADLAAMKG YDGLAAYLSE KALVEQFKDM SIAGNASGSL QTSSNYGGNS ENLSEDEIHL 660 KDTLAAYRTA ADAAARIQAA FRENSLKLKA KAVQYSTPEA EARGIIAALK IQHAFRNYDT 720 RKKIKAAARI QYRFRTWKMR QEFLSLRRQA IKIQAAFRGF QVRRQYRKVL WSVGVLEKAV 780 LRWRLKRRGL RGLNVAPVEV DVDQKQESDT EEDFYRASRK QAEERIERSV VRVQAMFRSK 840 KAQEEYSRMK LTHIEAKLEF EELLDPDSNM DS* |
| Expression -- Description ? help Back to Top | ||||||
|---|---|---|---|---|---|---|
| Source | Description | |||||
| Uniprot | TISSUE SPECIFICITY: Expressed in roots, stems, leaves, pollen, top of sepals and siliques. {ECO:0000269|PubMed:12218065, ECO:0000269|PubMed:14581622}. | |||||
| Functional Description ? help Back to Top | ||||||
|---|---|---|---|---|---|---|
| Source | Description | |||||
| UniProt | Transcription activator (PubMed:14581622). Binds to the DNA consensus sequence 5'-[ACG]CGCG[GTC]-3' (By similarity). Regulates transcriptional activity in response to calcium signals (Probable). Binds calmodulin in a calcium-dependent manner (By similarity). Involved in response to cold. Contributes together with CAMTA3 to the positive regulation of the cold-induced expression of DREB1A/CBF3, DREB1B/CBF1 and DREB1C/CBF2 (PubMed:28351986). {ECO:0000250|UniProtKB:Q8GSA7, ECO:0000269|PubMed:14581622, ECO:0000269|PubMed:28351986, ECO:0000305|PubMed:11925432}. | |||||
| Binding Motif ? help Back to Top | |||
|---|---|---|---|
| Motif ID | Method | Source | Motif file |
| MP00435 | DAP | Transfer from AT4G16150 | Download |
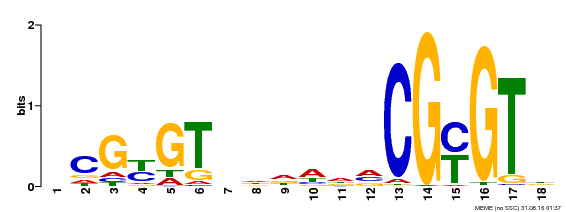
| |||
| Cis-element ? help Back to Top | |
|---|---|
| Source | Link |
| PlantRegMap | Prupe.1G122800.3.p |
| Regulation -- Description ? help Back to Top | ||||||
|---|---|---|---|---|---|---|
| Source | Description | |||||
| UniProt | INDUCTION: By heat shock, UVB, wounding, abscisic acid, H(2)O(2) and salicylic acid. {ECO:0000269|PubMed:12218065}. | |||||
| Regulation -- PlantRegMap ? help Back to Top | ||||||
|---|---|---|---|---|---|---|
| Source | Upstream Regulator | Target Gene | ||||
| PlantRegMap | Retrieve | Retrieve | ||||
| Annotation -- Protein ? help Back to Top | |||||||
|---|---|---|---|---|---|---|---|
| Source | Hit ID | E-value | Description | ||||
| Refseq | XP_020409879.1 | 0.0 | calmodulin-binding transcription activator 5 isoform X1 | ||||
| Swissprot | O23463 | 0.0 | CMTA5_ARATH; Calmodulin-binding transcription activator 5 | ||||
| TrEMBL | A0A251QWH2 | 0.0 | A0A251QWH2_PRUPE; Uncharacterized protein | ||||
| STRING | XP_008223308.1 | 0.0 | (Prunus mume) | ||||
| Best hit in Arabidopsis thaliana ? help Back to Top | ||||||
|---|---|---|---|---|---|---|
| Hit ID | E-value | Description | ||||
| AT4G16150.1 | 0.0 | calmodulin binding;transcription regulators | ||||
| Link Out ? help Back to Top | |
|---|---|
| Phytozome | Prupe.1G122800.3.p |
| Publications ? help Back to Top | |||
|---|---|---|---|
|
|||



